Research
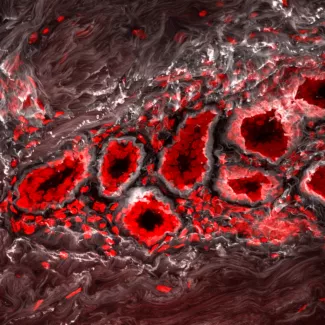
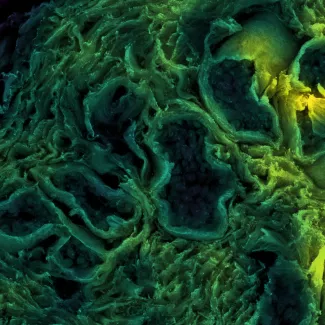
The extracellular matrix (ECM), the noncellular component of the microenvironment, influences cell growth, survival, migration and tissue-specific differentiation through a repertoire of receptors including integrins, syndecans and discoidin receptors. My group is exploring the molecular mechanisms whereby ECM receptors modulate cell fate and tumorigenesis. We investigate how mechanical and topological properties of the ECM, which are related to its composition and organization, alter integrin expression and function to modify stem cell fate and tumor progression.
One part of our research program focuses on clarifying how mechanical force (ECM stiffness and cell contractility) modulate breast tumor progression and treatment efficacy with a focus on signaling and epigenetics using two and three dimensional (2D/3D) monolayer and organotypic culture models, xenograft/syngeneic and transgenic mouse models and clinical specimens and a repertoire of approaches to measure and manipulate mechanical force. The second part of our research is directed towards characterizing the role of and molecular mechanisms whereby mechanical force regulates human embryonic stem cell fate using 2D/3D natural and synthetic matrices and a force reactor with a focus on adhesion signaling and epigenetics.
